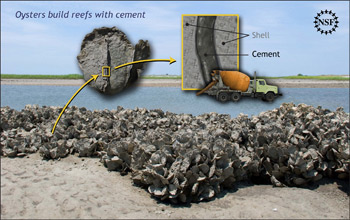
 |
| Image from NSF website. |
Calcium carbonate is a chemical compound with the chemical formula CaCO3. It is a common substance found in rock in all parts of the world, and is the main component of shells of marine organisms, snails, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime. It is commonly used medicinally as a calcium supplement or as an antacid.
The potential of the beneficial impacts of oysters and their calcium carbonate should not be ignored as a tool for fighting ocean acidification. In 1989, a researcher, Ken Simmons, introduced CaCO3 into the Whetstone Brook in Massachusetts. His hope was that the calcium carbonate would counter the acid in the stream from acid rain and save the trout that had ceased to spawn. His experiment was a success. This shows that CaCO3 can be added to neutralize the effects of acid rain in river ecosystems. Since the 1970s, such liming has been practiced on a large scale in Sweden to mitigate acidification and several thousand lakes and streams are limed repeatedly. Is it not logical step to think that the presence of large amounts of oyster shell could have a similar impact on a tidal estuary?
To see the original article on the NSF website you can click here. Oyster Cement Characterization Article.
The researchers presented their findings at the 2010 Annual Meeting of the American Chemical Society in Boston, and will publish their results in the Sept. 15, 2010, issue of the Journal of the American Chemical Society. To learn more go to glue abstract at The Journal of the American Chemical Society.

No comments:
Post a Comment